| Indian eyedrops: 8 people lost vision, 3 deaths |
| 送交者: 2023年03月24日19:33:34 于 [世界军事论坛] 发送悄悄话 |
|
|
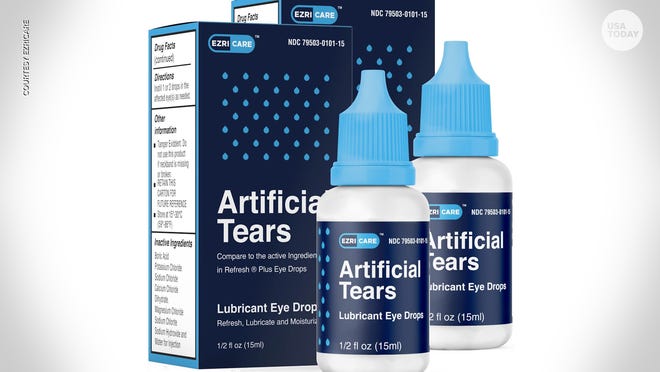
8 people with lost vision, 3 deaths: What we know about eyedrops recalled by the FDA Wyatte Grantham-Philips Wyatte Grantham-PhilipsUSA TODAY 3/24/2023 An outbreak of a drug-resistant bacteria strain tied to recalled eyedrops has now been linked to three deaths, among other serious injuries, according to the Centers for Disease Control and Prevention. Over recent months, the CDC and U.S. Food and Drug Administration have warned patients and clinicians to stop using EzriCare or Delsam Pharma’s Artificial Tears products. The products, which are manufactured by Global Pharma Healthcare based in India, were recalled in February over potential bacterial contamination – linked to the multistate outbreak of an extensively drug-resistant strain of Pseudomonas aeruginosa. In addition to the three deaths, an investigation into the outbreak confirmed eight reports of vision loss and four cases of eyeballs being surgically removed as of March 14, the CDC said. 'I want justice':Woman who lost her eye to infection sues recalled eyedrop maker EzriCare While immediate action is recommended for those who own the recalled artificial tears, consumers with eyedrops that are not under recall should feel safe continuing to use their products, health providers say. "Only users of the specific brands being recalled should be concerned due to the recent recall," Dr. Christopher Starr, clinical spokesperson for the American Academy of Ophthalmology, said in a statement sent to USA TODAY on Wednesday. "Before putting eyedrops in, everyone should double check the bottle’s label to be certain it isn’t one of these recalled products. But at this time there is no concern with using other eyedrops," Starr said. USA TODAY reached out to Global Pharma Healthcare for statement on Thursday. Here's what you need to know. More eyedrops are being recalled:FDA names two more brands being recalled due to health risks What else is under recall? Check out USA TODAY's searchable recall database; cars, food, consumer good and more. Deaths, vision loss in outbreak linked to Global Pharma artificial tearsThe outbreak of the drug-resistant strain of Pseudomonas aeruginosa linked to recalled products like EzriCare and Delsam Pharma’s Artificial Tears has reached 16 states, the CDC said. Sixty-eight patients had been identified as of March 14 – with more than half linked to four healthcare facility clusters, the CDC said. Multiple types of infections, including eye infections, have been associated with the outbreak. Three people died, eight reported vision loss and there were four cases of enucleation, the procedure where an eyeball is surgically removed. "Most patients reported using artificial tears," the CDC wrote, noting that more than 10 different brands of artificial tears were reported. EzriCare Artificial Tears was reported the most, according to the CDC. EzriCare's eyedrops are preservative-free. Preservative-free eyedrop bottles generally have a higher risk of contamination, Starr and Dr. Daniel Laroche, president of Advanced Eyecare of New York and clinical associate professor of ophthalmology at Mount Sinai's Icahn School of Medicine, told USA TODAY. "Without antimicrobial preservatives, bacteria and fungus can proliferate potentially leading to sight-threatening infections," Starr stated. "But because traditional preservatives in high doses (e.g. more than 4 drops per day) can be irritating to the ocular surface, we generally recommend preservative-free drops in single-use disposable containers for most people." 'Urgent threat':CDC warns against Candida auris, a drug-resistant fungus invading health care facilities
The drug-resistant bacterial strain of seen in this outbreak is officially called "carbapenem-resistant Pseudomonas aeruginosa with Verona integron-mediated metallo-β-lactamase and Guiana extended-spectrum-β-lactamase," or VIM-GES-CRPA, the CDC said. VIM-GES-CRPA is very rare. The strain "had never been reported in the United States prior to this outbreak," the CDC wrote. What is Pseudomonas aeruginosa?Pseudomonas is a bacteria that can be found in the environment, including in water and soil. There are multiple types of Pseudomonas – Pseudomonas aeruginosa causes the most infections in humans, according to the CDC. Pseudomonas aeruginosa can spread to people through contaminated surfaces, equipment, water and more. The bacteria can cause infections in the lungs, blood and other parts of the body. Pseudomonas aeruginosa "is a very dangerous bacteria because it could melt through the eye up to the cornea into the bloodstream pretty quickly," Laroche told USA TODAY. The infections are especially difficult to treat as Pseudomonas aeruginosa is "constantly finding new ways to avoid the effects of the antibiotics," the CDC notes. Tens of thousands of Pseudomonas aeruginosa infections in hospitalized patients have been reported over recent years, according to the CDC. People with weakened immune systems are particularly at risk. What's everyone talking about? Sign up for our trending newsletter to get the latest news of the day. Eyedrop safetyAgain, people with eyedrops that are not under recall should feel safe continuing to use their products as usual. But there are additional measures eyedrop consumers can take to stay safe in their everyday routines. "As a general rule of thumb, it is recommended to not use eyedrops that are past their expiration date and to keep the bottle tips clean and free of contamination," Starr said. "Always recap the bottle and keep in a clean location." Here are other safety tips that Starr and Laroche recommend:
If you get "a red eye or decreased vision, seek immediate care right away," Larcoche said. "The earlier you can get treatment, you can prevent a more serious complication." Similar rules apply to contact lens solutions, Starr said. Don't "reuse" or "top-off" the solution, as that can increase risks for infections. |
|
|
 |
|||||||||||||
|
 |
| 实用资讯 | |
|
|
| 一周点击热帖 | 更多>> |
| 一周回复热帖 |
| 历史上的今天:回复热帖 |
| 2022: | 俄军高级将领为什么接连阵亡——俄乌战 | |
| 2022: | 中国面临第三个战略机遇期 关键人物竟是 | |
| 2021: | 美媒:美国造船厂设备已严重老化 核潜艇 | |
| 2021: | 中国人不吃这一套, 谷歌翻译是,Chines | |
| 2020: | 武汉昨日新增确诊病例1例系医生 不排除 | |
| 2020: | Maatje Benassi 是美国外交警卫人员曾参 | |
| 2019: | 美国拟立法禁止进口中国制造的铁路列车 | |
| 2019: | 六十年前,当汉字被简化之后 | |
| 2018: | 环球时报:中国既坚定又冷静 打还是谈请 | |
| 2018: | 局座退休后练神功,升级为张半仙(多图 | |