Radiation Basics
Radiation is energy given off by matter in the form of rays or high-speed particles. All matter is composed ofatoms. Atoms are made up of various parts; the nucleus contains minute particles called protons andneutrons, and the atom's outer shell contains other particles called electrons. The nucleus carries a positive electrical charge, while the electrons carry a negative electrical charge. These forces within the atom work toward a strong, stable balance by getting rid of excess atomic energy (radioactivity). In that process, unstable nuclei may emit a quantity of energy, and this spontaneous emission is what we call radiation.
For additional information, see the following topics on this page:
As previously indicated, matter gives off energy (radiation) in two basic physical forms. One form of radiation is pure energy with no weight. This form of radiation — known as electromagnetic radiation — is like vibrating or pulsating rays or "waves" of electrical and magnetic energy. Familiar types of electromagnetic radiation include sunlight (cosmic radiation), x-rays, radar, and radio waves.
The other form of radiation — known as particle radiation — is tiny fast-moving particles that have both energy and mass (weight). This less-familiar form of radiation includes alpha particles, beta particles, andneutrons, as explained below.

Radioactive Decay
As previously indicated, large unstable atoms become more stable by emitting radiation to get rid of excess atomic energy (radioactivity). This radiation can be emitted in the form of positively charged alpha particles, negatively charged beta particles, gamma rays, or x-rays, as explained below.
Through this process — called radioactive decay — radioisotopes lose their radioactivity over time. This gradual loss of radioactivity is measured in half-lives. Essentially, a half-life of a radioactive material is the time it takes one-half of the atoms of a radioisotope to decay by emitting radiation. This time can range from fractions of a second (for radon-220) to millions of years (for thorium-232). When radioisotopes are used in medicine or industry, it is vital to know how rapidly they lose their radioactivity, in order to know the precise amount of radioisotope that is available for the medical procedure or industrial use.

Nuclear Fission
In some elements, the nucleus can split as a result of absorbing an additional neutron, through a process called nuclear fission. Such elements are called fissile materials. One particularly notable fissile material is uranium-235. This is the isotope that is used as fuel in commercial nuclear power plants.
When a nucleus fissions, it causes three important events that result in the release of energy. Specifically, these events are the release of radiation, release of neutrons (usually two or three), and formation of two new nuclei (fission products).

Ionizing Radiation
Radiation can be either ionizing or non-ionizing, depending on how it affects matter. Non-ionizing radiation includes visible light, heat, radar, microwaves, and radio waves. This type of radiation deposits energy in the materials through which it passes, but it does not have sufficient energy to break molecular bonds or remove electrons from atoms.
By contrast, ionizing radiation (such as x-rays and cosmic rays) is more energetic than non-ionizing radiation. Consequently, when ionizing radiation passes through material, it deposits enough energy to break molecular bonds and displace (or remove) electrons from atoms. This electron displacement creates two electrically charged particles (ions), which may cause changes in living cells of plants, animals, and people.
Ionizing radiation has a number of beneficial uses. For example, we use ionizing radiation in smoke detectors and to treat cancer or sterilize medical equipment. Nonetheless, ionizing radiation is potentially harmful if not used correctly. Consequently, the U.S. Nuclear Regulatory Commission (NRC) strictly regulates commerical and institutional uses of nuclear materials, including the following five major types of ionizing radiation:
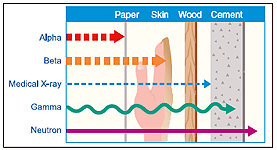

Alpha Particles
Alpha particles are charged particles, which are emitted from naturally occurring materials (such as uranium, thorium, and radium) and man-made elements (such as plutonium and americium). These alpha emitters are primarily used (in very small amounts) in items such as smoke detectors.
In general, alpha particles have a very limited ability to penetrate other materials. In other words, these particles of ionizing radiation can be blocked by a sheet of paper, skin, or even a few inches of air. Nonetheless, materials that emit alpha particles are potentially dangerous if they are inhaled or swallowed, but external exposure generally does not pose a danger.